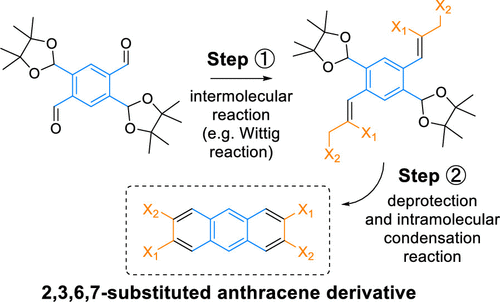
Our work reporting a double ring-closing approach for the synthesis of anthracene derivatives has been accepted for publication in the Journal of Organic Chemistry.
In the paper, we report a method for the synthesis of 2,3,6,7-substituted anthracene derivatives, one of the most challenging anthracene substitution patterns to obtain. The method is exemplified by the preparation of 2,3,6,7-anthracenetetracarbonitrile and employs a newly developed, stable, protected 1,2,4,5-benzenetetracarbaldehyde as the precursor. The precursor can be obtained in two scalable synthetic steps from 2,5-dibromoterephthalaldehyde and is converted into the anthracene derivative by a double intermolecular Wittig reaction under very mild conditions, followed by a deprotection and intramolecular double ring-closing condensation reaction.
Congratulations to first authors Birgit Meindl and Katharina Pfennigbauer!
B. Meindl, K. Pfennigbauer, B. Stöger, M. Heeney, F. Glöcklhofer,* Double Ring-Closing Approach for the Synthesis of 2,3,6,7-Substituted Anthracene Derivatives, J. Org. Chem. 2020, 85, 8240-8244