PREPRINT(S)
Concealed antiaromaticity [Preprint link, open access]
F. Glöcklhofer*
ChemRxiv 2023, preprint, DOI:10.26434/chemrxiv-2023-hnl0w-v2
The literature reports numerous molecules claimed to be antiaromatic because of a formal 4n π-electron system. However, this neglects the actual local aromaticity of the molecules, which often feature multiple subunits with [4n+2] π-electrons besides the formal 4n π-electron system. This has led to considerable criticism from those who believe that the term antiaromatic should not be used for any molecule with a formal 4n π-electron system but should be reserved for truly antiaromatic molecules. To reconcile the different viewpoints, the concept of concealed antiaromaticity is introduced here. Concealed antiaromaticity acknowledges that many molecules claimed to be antiaromatic are not truly antiaromatic, but they can exhibit behaviour under certain conditions that would normally be expected for antiaromatic molecules. Three types of concealed antiaromaticity are distinguished based on the conditions under which the molecules can behave like antiaromatic molecules: concealed antiaromaticity revealable in redox reactions (Type I-CA), upon photoexcitation (Type II-CA), and in intermolecular interactions (Type III-CA). The concept of concealed antiaromaticity will enable the rational design of molecules that show the desirable properties of antiaromatic molecules under the different conditions, with applications from organic electronics to photoresponsive materials, while avoiding the low stability of truly antiaromatic molecules.
JOURNAL ARTICLES
20 corresponding author papers shown in frames
2024
39. Excitonic organic materials for photochemical and optoelectronic applications: general discussion [Link]
C. M. Aitchison, K. Albrecht, K. Awaga, K. Bergmann, J. Calbo, J. Cameron, J. Clark, M. Collins, P. Data, P. dos Santos, T. Fujigaya, T. Fujino, A. Fukazawa, F. Glöcklhofer, X. Guo, M. Heeney, Z. M. Hudson, Y. Ie, W. Ishii, C. K. Luscombe, R. Marcilla, T. Matsuo, S. Miyazaki, S. Nakagawa, T. Nakanishi, N. Nakatsuka, H. Nishide, Y. Sasaki, B. C. Schroeder, M. Singh, P. Skabara, Y. Takeda, Y. Tanaka, Y. Tani, Y. Tsuchiya, Y. Tsutsui, T. Uematsu, G. Xie, N. Yanai
38. Organic batteries: general discussion [Link]
C. M. Aitchison, K. Albrecht, K. Awaga, J. Cameron, P. Data, A. Fukazawa, F. Glöcklhofer, Y. Ie, C. K. Luscombe, R. Marcilla, N. Nakatsuka, H. Nishide, B. C. Schroeder, M. Singh, P. Skabara, Y. Takeda, Y. Tani, T. Uematsu, G. Xie, D. Yadav, Y. Yakiyama
37. Organic neuromorphics and bioelectronics: general discussion [Link]
C. M. Aitchison, K. Albrecht, K. Awaga, J. Cameron, P. Data, F. Glöcklhofer, X. Guo, M. Heeney, Z. M. Hudson, Y. Ie, C. K. Luscombe, T. Matsuo, T. Nakanishi, N. Nakatsuka, H. Nishide, Y. Sasaki, B. C. Schroeder, M. Singh, P. Skabara, Y. Takeda, Y. Tani, L. Torsi, Y. Tsuchiya, T. Uematsu, D. Yadav, N. Yanai
36. Reducing Undesired Solubility of Squarephaneic Tetraimide for Use as an Organic Battery Electrode Material [Link, open access]
B. Ding, M. Bhosale, T. Bennett, M. Heeney, F. Plasser, B. Esser,* F. Glöcklhofer*
Locally aromatic alkyl-N-substituted squarephaneic tetraimide (SqTI) conjugated macrocycles are four-electron reducible, owing to global aromaticity and presumed global Baird aromaticity of the dianion and tetraanion states, respectively. However, their good solubility inhibits their application as a battery electrode material. By applying sidechain removal as a strategy to reduce SqTI solubility, we report the development of its unsubstituted derivative SqTI-H, which was obtained directly from squarephaneic tetraanhydride by facile treatment with hexamethyldisilazane and MeOH. Compared to alkyl-N-substituted SqTI-Rs, SqTI-H exhibited further improved thermal stability and low neutral state solubility in most common organic solvents, owing to computationally demonstrated hydrogen-bonding capabilities emanating from each imide position on SqTI-H. Reversible solid state electrochemical reduction of SqTI-H to the globally aromatic dianion state was also observed at -1.25 V vs. Fc/Fc+, which could be further reduced in two stages. Preliminary testing of SqTI-H in composite electrodes for lithium-organic half cells uncovered imperfect cycling performance, which may be explained by persistent solubility of reduced states, necessitating further optimisation of electrode fabrication procedures to attain maximum performance.
2023
35. Functionalisation of conjugated macrocycles with type I and II concealed antiaromaticity via cross-coupling reactions [Link, open access]
T. L. R. Bennett, A. V. Marsh, J. M. Turner, F. Plasser, M. Heeney, F. Glöcklhofer*
Mol. Syst. Des. Eng. 2023, 8, 713-720. DOI:10.1039/d3me00045a
Conjugated macrocycles can exhibit concealed antiaromaticity; that is, despite not being antiaromatic, under specific circumstances, they can display properties typically observed in antiaromatic molecules due to their formal macrocyclic 4n π-electron system. Paracyclophanetetraene (PCT) and its derivatives are prime examples of macrocycles exhibiting this behaviour. In redox reactions and upon photoexcitation, they have been shown to behave like antiaromatic molecules (requiring type I and II concealed antiaromaticity, respectively), with such phenomena showing potential for use in battery electrode materials and other electronic applications. However, further exploration of PCTs has been hindered by the lack of halogenated molecular building blocks that would permit their integration into larger conjugated molecules by cross-coupling reactions. Here, we present two dibrominated PCTs, obtained as a mixture of regioisomers from a three-step synthesis, and demonstrate their functionalisation via Suzuki cross-coupling reactions. Optical, electrochemical, and theoretical studies reveal that aryl substituents can subtly tune the properties and behaviour of PCT, showing that this is a viable strategy in further exploring this promising class of materials.
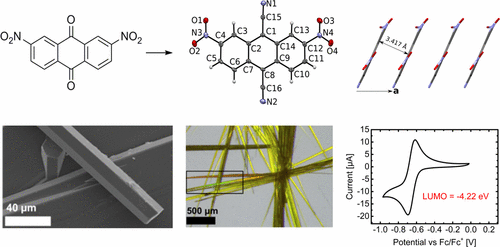
34. 1D slipped stacking microribbon-like crystals based on 6,13-dicyanopentacene for ambipolar charge transport [Link, open access] [SciEngine link, open access] [Repository link, open access]
Z. Wang, Y. Hu, Y. Xie, F. Qie, J. Guo, L. Zhang, C. Shi,* F. Glöcklhofer,* Y. Zhen*
Sci. China Mater. 2023, 66, 2429-2435. DOI:10.1007/s40843-022-2364-3
Featuring small charge transport scattering, mesoscale size, and easy fabrication, one-dimensional self-assembled micro/nanomaterials (1D-MNMs) based on organic π-conjugated systems can be facilely incorporated into integrated microcircuits. Although tremendous progress has been made in 1D-MNMs based on p- or n-channel organic semiconductors, examples of 1D-MNMs based on ambipolar organic semiconductors are scarce. Herein, we achieved a novel 1D-MNM based on 6,13-dicyanopentacene (DCP) with a 1D slipped stacking motif using the physical vapor transport method. The DCP-based 1D-MNM showed outstanding, well-balanced ambipolar charge transport with electron and hole mobilities of up to 0.34 and 0.38 cm2 V−1 s−1, respectively, which are among the best ambipolar transport characteristics of 1D-MNMs. Furthermore, a complementary inverter based on the ambipolar 1D-MNM of DCP was also constructed with a gain of up to 7, indicating potential application in organic logic circuits.
2022
33. Squarephaneic Tetraanhydride: A Conjugated Square-Shaped Cyclophane for the Synthesis of Porous Organic Materials [Link, open access] [Preprint link, open access]
S. Eder,# B. Ding,# D. B. Thornton, D. Sammut, A. J. P. White, F. Plasser, I. E. L. Stephens, M. Heeney, S. Mezzavilla, F. Glöcklhofer* (# contributed equally)
Angew. Chem., Int. Ed. 2022, 61, e202212623. DOI:10.1002/anie.202212623
Angew. Chem. 2022, 134, e202212623. DOI:10.1002/ange.202212623
Aromatic carboxylic anhydrides are ubiquitous building blocks in organic materials chemistry and have received considerable attention in the synthesis of organic semiconductors, pigments, and battery electrode materials. Here we extend the family of aromatic carboxylic anhydrides with a unique new member, a conjugated cyclophane with four anhydride groups. The cyclophane is obtained in a three-step synthesis and can be functionalised efficiently, as shown by the conversion into tetraimides and an octacarboxylate. Crystal structures reveal the high degree of porosity achievable with the new building block. Excellent electrochemical properties and reversible reduction to the tetraanions are shown for the imides; NMR and EPR measurements confirm the global aromaticity of the dianions and evidence the global Baird aromaticity of the tetraanions. Considering the short synthesis and unique properties, we expect widespread use of the new building block in the development of organic materials.
32. Post-polymerisation approaches for the rapid modification of conjugated polymer properties [Link, open access]
M. Rimmele, F. Glöcklhofer,* M. Heeney*
Post-polymerisation functionalisation provides a facile and efficient way for the introduction of functional groups on the backbone of conjugated polymers. Using post-polymerisation functionalisation approaches, the polymer chain length is usually not affected, meaning that the resulting polymers only differ in their attached functional groups or side chains, which makes them particularly interesting for investigating the influence of the different groups on the polymer properties. For such functionalisations, highly efficient and selective reactions are needed to avoid the formation of complex mixtures or permanent defects in the polymer backbone. A variety of suitable synthetic approaches and reactions that fulfil these criteria have been identified and reported. In this review, a thorough overview is given of the post-polymerisation functionalisations reported to date, with the methods grouped based on the type of reaction used: cycloaddition, oxidation/reduction, nucleophilic aromatic substitution, or halogenation and subsequent cross-coupling reaction. Instead of modifications on the aliphatic side chains of the conjugated polymers, we focus on modifications directly on the conjugated backbones, as these have the most pronounced effect on the optical and electronic properties. Some of the discussed materials have been used in applications, ranging from solar cells to bioelectronics. By providing an overview of this versatile and expanding field for the first time, we showcase post-polymerisation functionalisation as an exciting pathway for the creation of new conjugated materials for a range of applications.
31. [2.2.2.2]Paracyclophanetetraenes (PCTs): cyclic structural analogues of poly(p-phenylene vinylene)s (PPVs) [version 2; peer review: 2 approved] [Link, open access]
M. Pletzer, F. Plasser, M. Rimmele, M. Heeney, F. Glöcklhofer*
Open Res. Europe 2022, 1, 111. DOI:10.12688/openreseurope.13723.2
Poly(p-phenylene vinylene)s (PPVs) and [2.2.2.2]paracyclophanetetraene (PCT) are both composed of alternating π-conjugated para-phenylene and vinylene units. However, while the former constitute a class of π-conjugated polymers that has been used in organic electronics for decades, the latter is a macrocycle that only recently revealed its potential for applications such as organic battery electrodes. The cyclic structure endows PCT with unusual properties, and further tuning of these may be required for specific applications. In this article, we adopt an approach often used for tuning the properties of PPVs, the introduction of alkoxy (or alkylthio) substituents at the phenylene units, for tuning the optoelectronic properties of PCT. The resulting methoxy- and methylthio-substituted PCTs, obtained by Wittig cyclisation reactions, are studied by UV-vis absorption, photoluminescence, and cyclic voltammetry measurements, and investigated computationally using the visualisation of chemical shielding tensors (VIST) method. The measurements show that substitution leads to slight changes in terms of absorption/emission energies and redox potentials while having a pronounced effect on the photoluminescence intensity. The computations show the effect of the substituents on the ring currents and chemical shielding and on the associated local and global (anti)aromaticity of the macrocycles, highlighting the interplay of local and global aromaticity in various electronic states. The study offers interesting insights into the tuneability of the properties of this versatile class of π-conjugated macrocycles.
2021
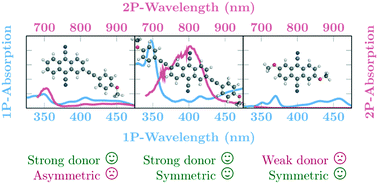
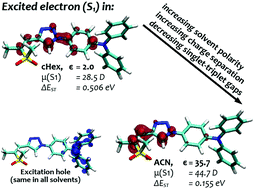
30. Excited-state symmetry breaking in 9,10-dicyanoanthracene-based quadrupolar molecules: the effect of donor-acceptor branch length [Link, open access]
Z. Szakács, F. Glöcklhofer, F. Plasser, E. Vauthey*
Phys. Chem. Chem. Phys. 2021, 23, 15150-15158. DOI:10.1039/D1CP02376D
Excited-state symmetry breaking is investigated in a series of symmetric 9,10-dicyanoanthracenes linked to electron-donating groups on the 2 and 6 positions via different spacers, allowing for a tuning of the length of the donor-acceptor branches. The excited-state properties of these compounds are compared with their dipolar single-branch analogues. The changes in electronic structure upon their optical excitation are monitored by transient electronic spectroscopy in the visible and near-infrared regions as well as by transient vibrational spectroscopy in the mid-infrared. Our results reveal that, with the shortest branches, electronic excitation remains distributed almost symmetrically over the molecule even in polar environments. Upon increasing the donor-acceptor distance, excitation becomes unevenly distributed and, with the longest one, it fully localises on one branch in polar solvents. The influence of the branch length on the propensity of quadrupolar dyes to undergo excited-state symmetry breaking is rationalised in terms of the balance between interbranch coupling and solvation energy.
29. Functional group introduction and aromatic unit variation in a set of π‑conjugated macrocycles: revealing the central role of local and global aromaticity [Link, open access] [Preprint link, open access]
M. Rimmele, W. Nogala, M. Seif-Eddine, M. M. Roessler, M. Heeney, F. Plasser,* F. Glöcklhofer*
Org. Chem. Front. 2021, 8, 4730-4745. DOI:10.1039/D1QO00901J
π-Conjugated macrocycles are molecules with unique properties that are increasingly exploited for applications and the question of whether they can sustain global aromatic or antiaromatic ring currents is particularly intriguing. However, there are only a small number of experimental studies that investigate how the properties of π-conjugated macrocycles evolve with systematic structural changes. Here, we present such a systematic experimental study of a set of [2.2.2.2]cyclophanetetraenes, all with formally Hückel antiaromatic ground states, and combine it with an in-depth computational analysis. The study reveals the central role of local and global aromaticity for rationalizing the observed optoelectronic properties, ranging from extremely large Stokes shifts of up to 1.6 eV to reversible fourfold reduction, a highly useful feature for charge storage/accumulation applications. A recently developed method for the visualization of chemical shielding tensors (VIST) is applied to provide unique insight into local and global ring currents occurring in different planes along the macrocycle. Conformational changes as a result of the structural variations can further explain some of the observations. The study contributes to the development of structure–property relationships and molecular design guidelines and will help to understand, rationalize, and predict the properties of other π-conjugated macrocycles. It will also assist in the design of macrocycle-based supramolecular elements with defined properties.
28. Visualisation of Chemical Shielding Tensors (VIST) to Elucidate Aromaticity and Antiaromaticity [Link, open access] [Preprint link, open access]
F. Plasser,* F. Glöcklhofer
Eur. J. Org. Chem. 2021, 2021, 2529-2539. DOI:10.1002/ejoc.202100352
Aromaticity is a central concept in chemistry, pervading areas from biochemistry to materials science. Recently, chemists also started to exploit intricate phenomena such as the interplay of local and global (anti)aromaticity or aromaticity in non‐planar systems and three dimensions. These phenomena pose new challenges in terms of our fundamental understanding and the practical visualisation of aromaticity. To overcome these challenges, a method for the visualisation of chemical shielding tensors (VIST) is developed here that allows for a 3D visualisation with quantitative information about the local variations and anisotropy of the chemical shielding. After exemplifying the method in different planar hydrocarbons, we study two non‐planar macrocycles to show the unique benefits of the VIST method for molecules with competing pi‐conjugated systems and conclude with a norcorrole dimer showing clear evidence of through‐space aromaticity. We believe that the VIST method will be a highly valuable addition to the computational toolbox.
27. Tetradiketone macrocycle for divalent aluminium ion batteries [Link, open access]
D.-J. Yoo, M. Heeney, F. Glöcklhofer,* J. W. Choi*
Contrary to early motivation, the majority of aluminium ion batteries developed to date do not utilise multivalent ion storage; rather, these batteries rely on monovalent complex ions for their main redox reaction. This limitation is somewhat frustrating because the innate advantages of metallic aluminium such as its low cost and high air stability cannot be fully taken advantage of. Here, we report a tetradiketone macrocycle as an aluminium ion battery cathode material that reversibly reacts with divalent (AlCl2+) ions and consequently achieves a high specific capacity of 350 mAh g−1 along with a lifetime of 8000 cycles. The preferred storage of divalent ions over their competing monovalent counterparts can be explained by the relatively unstable discharge state when using monovalent AlCl2+ ions, which exert a moderate resonance effect to stabilise the structure. This study opens an avenue to realise truly multivalent aluminium ion batteries based on organic active materials, by tuning the relative stability of discharged states with carrier ions of different valence states.
26. The influence of alkyl group regiochemistry and backbone fluorination on the packing and transistor performance of N-cyanoimine functionalised indacenodithiophenes [Link, open access]
T. Hodsden, K. J. Thorley, A. Basu, A. J. P. White, C. Wang, W. Mitchell, F. Glöcklhofer, T. D. Anthopoulos, M. Heeney*
The synthesis of two novel n-type molecular organic semiconductors based on a fluorinated indacenodithiophene core in combination with an electron withdrawing N-cyanoimine group is reported, and the influence of the regiochemistry of the solubilizing sidechain is investigated. The N-cyanoimine is confirmed to be a strongly electron accepting group, which in combination with the core fluorination resulted in high electron affinities for both materials. Single crystal analysis demonstrated that whilst both materials arrange in ordered slipped stacks with close π-π stacking distances (~ 3.40 Å), significant differences in electron transfer integrals for the two regioisomers were observed, relating to differences in relative molecular displacement along the π-stacking direction. Organic thin-film transistors fabricated via blade-coating displayed electron mobility up to 0.13 cm2 V-1 s-1 for the isomer with the larger transfer integral.
25. One‐step Six‐fold Cyanation of Benzothiadiazole Acceptor Units for Air‐Stable High‐Performance n‐Type Organic Field‐Effect Transistors [Link, open access]
P. Kafourou, B. Park, J. Luke, L. Tan, J. Panidi, F. Glöcklhofer, J. Kim, T. D. Anthopoulos, J.-S. Kim, K. Lee, S. Kwon,* M. Heeney*
Angew. Chem., Int. Ed. 2021, 60, 5970-5977. DOI:10.1002/anie.202013625
Angew. Chem. 2021, 133, 6035-6042. DOI:10.1002/ange.202013625
We report a new high electron affinity acceptor end group for organic semiconductors, 2,1,3‐benzothiadiazole‐4,5,6‐tricarbonitrile (TCNBT). An n‐type organic semiconductor with an indacenodithiophene (IDT) core and TCNBT end groups was synthesized by a six‐fold nucleophilic substitution with cyanides on a fluorinated precursor, itself prepared by a direct arylation approach. This one‐step chemical modification was found to significantly impact the molecular properties: the fluorinated precursor, TFBT IDT, a poor ambipolar semiconductor, was converted into TCNBT IDT, a good n‐type semiconductor. The highly electron‐deficient end group TCNBT dramatically decreased the energy of the highest occupied and lowest unoccupied molecular orbitals (HOMO/LUMO) compared to the fluorinated analogue and improved the molecular orientation when utilized in n‐type organic field‐effect transistors (OFETs). Solution‐processed OFETs based on TCNBT IDT exhibited a charge carrier mobility of up to µe ≈ 0.15 cm2 V‐1 s‐1 with excellent ambient stability for 100 hours, highlighting the benefits of the cyanated end group and the synthetic approach.
2020
24. Multibranched aliphatic side chains for π-conjugated polymers with a high density of ‘unshielded’ aromatics [Link, open access]
S. Wang, J. Shaw, Y. Han, Z. Fei, F. Glöcklhofer,* M. Heeney*
The synthesis of strongly solubilising multibranched aliphatic side chains for π-conjugated polymers is reported. The solubilising capability of the side chains and their effect on the polymer properties are studied on the example of copolymers composed of up to six unsubstituted, ‘unshielded’ thiophene units per side chain-substituted naphthalene diimide unit.
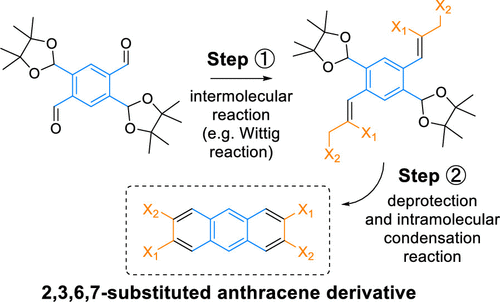
23. Double Ring-Closing Approach for the Synthesis of 2,3,6,7-Substituted Anthracene Derivatives [Link] [Preprint link, open access]
B. Meindl,# K. Pfennigbauer,# B. Stöger, M. Heeney, F. Glöcklhofer* (# contributed equally)
J. Org. Chem. 2020, 85, 8240-8244. DOI:10.1021/acs.joc.0c00826
A method for the synthesis of 2,3,6,7-substituted anthracene derivatives, one of the most challenging anthracene substitution patterns to obtain, is presented. The method is exemplified by the preparation of 2,3,6,7-anthracenetetracarbonitrile and employs a newly developed, stable, protected 1,2,4,5-benzenetetracarbaldehyde as the precursor. The precursor can be obtained in two scalable synthetic steps from 2,5-dibromoterephthalaldehyde and is converted into the anthracene derivative by a double intermolecular Wittig reaction under very mild conditions, followed by a deprotection and intramolecular double ring-closing condensation reaction.
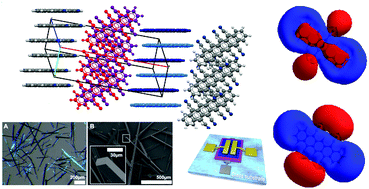
22. Switching between Local and Global Aromaticity in a Conjugated Macrocycle for High-Performance Organic Sodium-Ion Battery Anodes [Link, open access]
S. Eder,# D.-J. Yoo,# W. Nogala, M. Pletzer, A. Santana Bonilla, A. J. P. White, K. E. Jelfs, M. Heeney, J. W. Choi,* F. Glöcklhofer* (# contributed equally)
Angew. Chem., Int. Ed. 2020, 59, 12958-12964. DOI:10.1002/anie.202003386
Angew. Chem. 2020, 132, 13058-13064. DOI:10.1002/ange.202003386
Aromatic organic compounds can be used as electrode materials in rechargeable batteries and are expected to advance the development of both anode and cathode materials for sodium‐ion batteries (SIBs). However, most aromatic organic compounds assessed as anode materials in SIBs to date exhibit significant degradation issues under fast‐charge/discharge conditions and unsatisfying long‐term cycling performance. Now, a molecular design concept is presented for improving the stability of organic compounds for battery electrodes. The molecular design of the investigated compound, [2.2.2.2]paracyclophane‐1,9,17,25‐tetraene (PCT), can stabilize the neutral state by local aromaticity and the doubly reduced state by global aromaticity, resulting in an anode material with extraordinarily stable cycling performance and outstanding performance under fast‐charge/discharge conditions, demonstrating an exciting new path for the development of electrode materials for SIBs and other types of batteries.
21. Hydrothermal Generation of Conjugated Polymers Using the Example of Pyrrone Polymers and Polybenzimidazoles [Link, open access]
M. J. Taublaender, S. Mezzavilla, S. Thiele, F. Glöcklhofer, M. M. Unterlass*
Angew. Chem., Int. Ed. 2020, 59, 15050-15060. DOI:10.1002/anie.202000367
Angew. Chem. 2020, 132, 15160-15171. DOI:10.1002/ange.202000367
Various polyimides and polyamides have recently been prepared via hydrothermal synthesis in nothing but H2O under high‐pressure and high‐temperature conditions. However, none of the prepared polymers feature a truly conjugated polymer backbone. Here, we report on an expansion of the synthetic scope of this straightforward and inherently environmentally friendly polymerization technique to the generation of conjugated polymers. Selected representatives of two different polymer classes, pyrrone polymers and polybenzimidazoles, were generated hydrothermally. We present a mechanistic discussion of the polymer formation process as well as an electrochemical characterization of the most promising product.
20. Core Fluorination Enhances Solubility and Ambient Stability of an IDT‐Based n‐Type Semiconductor in Transistor Devices [Link] [Repository link, open access]
T. Hodsden, K. J. Thorley, J. Panidi, A. Basu, A. V. Marsh, H. Dai, A. J. P. White, C. Wang, W. Mitchell, F. Glöcklhofer, T. D. Anthopoulos,* M. Heeney*
Adv. Funct. Mater. 2020, 30, 2000325. DOI:10.1002/adfm.202000325
The synthesis of a novel fluorinated n‐type small molecule based on an indacenodithiophene core is reported. Fluorination is found to have a significant impact on the physical properties, including a surprisingly dramatic improvement in solubility, in addition to effectively stabilizing the lowest‐unoccupied molecular orbital energy (−4.24 eV). Single‐crystal analysis and density functional theory calculations indicate the improved solubility can be attributed to backbone torsion resulting from the positioning of the fluorine group in close proximity to the strongly electron‐withdrawing dicyanomethylene group. Organic thin‐film transistors made via blade coating display high electron mobility (up to 0.49 cm2 V−1 s−1) along with good retention of performance in ambient conditions.
2019
19. Effect of Symmetric and Asymmetric Substitution on the Optoelectronic Properties of 9,10-Dicyanoanthracene [Link] [Preprint link, open access]
F. Glöcklhofer,*# A. Rosspeintner,*# P. Pasitsuparoad, S. Eder, J. Fröhlich, G. Angulo, E. Vauthey, F. Plasser* (# contributed equally)
Mol. Syst. Des. Eng. 2019, 4, 951-961. DOI:10.1039/c9me00040b
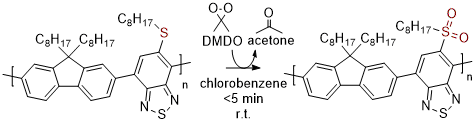
18. Fast and selective post-polymerization modification of conjugated polymers using dimethyldioxirane [Link, open access] [Preprint link, open access]
E. Reichsöllner, A. Creamer, S. Cong, A. Casey, S. Eder, M. Heeney, F. Glöcklhofer*
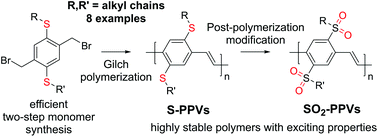
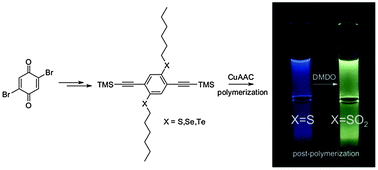
17. Thioalkyl- and Sulfone-Substituted Poly(p-Phenylene Vinylene)s [Link] [Preprint link, open access]
M. Rimmele, K. Ableidinger, A. V. Marsh, N. J. Cheetham, M. J. Taublaender, A. Buchner, J. Prinz, J. Fröhlich, M. M. Unterlass, M. Heeney, F. Glöcklhofer*
2018
16. Green and Rapid Hydrothermal Crystallization and Synthesis of Fully Conjugated Aromatic Compounds [Link, open access]
M. J. Taublaender, F. Glöcklhofer, M. Marchetti-Deschmann, M. M. Unterlass*
Angew. Chem., Int. Ed. 2018, 57, 12270-12274. DOI:10.1002/anie.201801277
15. Synthesis of 1,2,5,6- and 1,4,5,8-anthracenetetrone: Building blocks for π-conjugated small molecules and polymers [Link, open access] [Preprint link, open access]
F. Glöcklhofer,* B. Stöger, J. Fröhlich
Synth. Commun. 2018, 48, 2358-2365. DOI:10.1080/00397911.2018.1483027
2017
14. Extending the Scope of a New Cyanation: Design and Synthesis of an Anthracene Derivative with an Exceptionally Low LUMO Level and Improved Solubility [Link, open access]
F. Glöcklhofer,* A. J. Morawietz, B. Stöger, M. M. Unterlass, J. Fröhlich
13. Charge-transfer states in triazole linked donor–acceptor materials: strong effects of chemical modification and solvation [Link, open access]
P. Kautny, F. Glöcklhofer, T. Kader, J.-M. Mewes, B. Stöger, J. Fröhlich, D. Lumpi,* F. Plasser*
Phys. Chem. Chem. Phys. 2017, 19, 18055-18067. DOI:10.1039/C7CP01664F
12. Dicyano- and tetracyanopentacene: foundation of an intriguing new class of easy-to-synthesize organic semiconductors [Link, open access]
F. Glöcklhofer,* A. Petritz, E. Karner, M. J. Bojdys, B. Stadlober, J. Fröhlich, M. M. Unterlass
J. Mater. Chem. C 2017, 5, 2603-2610. DOI:10.1039/C7TC00143F
11. Using Dicyanoanthracene Triflates as Superior Precursors: Modifying Properties by Sterically Hindered Aryl Substituents [Link] [Repository link, open access]
F. Glöcklhofer,* P. Kautny, P. Fritz, B. Stöger, J. Fröhlich
10. Thiophene ring-fragmentation reactions: Principles and scale-up towards NLO materials [Link] [Repository link, open access]
D. Lumpi,* J. Steindl, S. Steiner, V. Carl, P. Kautny, M. Schön, F. Glöcklhofer, B. Holzer, B. Stöger, E. Horkel, C. Hametner, G. Reider, M. D. Mihovilovic, J. Fröhlich
Tetrahedron 2017, 73, 472-480. DOI:10.1016/j.tet.2016.12.025
2016
9. A Versatile One-Pot Access to Cyanoarenes from ortho- and para-Quinones: Paving the Way for Cyanated Functional Materials [Link] [Repository link, open access]
F. Glöcklhofer,* M. Lunzer, B. Stöger, J. Fröhlich
Chem. - Eur. J. 2016, 22, 5173-5180. DOI:10.1002/chem.201600004
8. cis,trans,cis-1,2,3,4-Tetrakis[2-(ethylsulfanyl)phenyl]cyclobutane [Link, open access]
B. Sohr, F. Glöcklhofer, B. Stöger, M. Weil, J. Fröhlich
2013 - 2015
7. Facile Synthesis of Cyanoarenes from Quinones by Reductive Aromatization of Cyanohydrin Intermediates [Link] [Repository link, open access]
F. Glöcklhofer,* M. Lunzer, J. Fröhlich
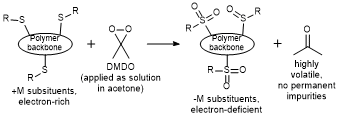
6. Towards continuous junction (CJ) organic electronic devices: Fast and clean post-polymerization modification by oxidation using dimethyldioxirane (DMDO) [Link] [Repository link, open access]
F. Glöcklhofer, D. Lumpi,* M. Kohlstädt, O. Yurchenko, U. Würfel, J. Fröhlich
React. Funct. Polym. 2015, 86, 16-26. DOI:10.1016/j.reactfunctpolym.2014.10.006
5. Multigram synthesis of bis[(trimethylsilyl) ethynyl]benzenes suitable for post-polymerization modification [Link] [Author version, open access]
F. Glöcklhofer, D. Lumpi,* B. Stöger, J. Fröhlich
4. Crystal structure of trans-1,4-bis[(trimethylsilyl) oxy]cyclohexa-2,5-diene-1,4-dicarbonitrile [Link, open access]
F. Glöcklhofer, J. Fröhlich, B. Stöger, M. Weil*
Acta Crystallogr., Sect. E 2014, 70, 77-79. DOI:10.1107/S1600536814014251
3. Crystal structures of 2,5-diazido-1,4-phenylene diacetate and 2,5-diazido-1,4-phenylene dibutyrate [Link, open access]
F. Glöcklhofer, J. Fröhlich, B. Stöger, M. Weil*
Acta Crystallogr., Sect. E 2014, 70, 39-42. DOI:10.1107/S1600536814013762
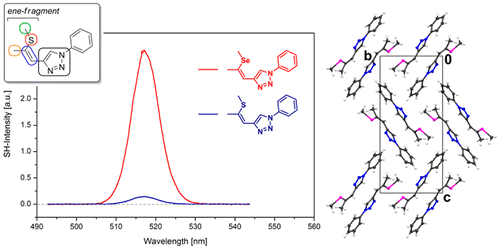
2. Systematic Investigations on 1,2,3-Triazole-Based Compounds Capable of Second Harmonic Generation [Link] [Repository link, open access]
D. Lumpi,* F. Glöcklhofer, B. Holzer, B. Stöger, C. Hametner, G. A. Reider, J. Fröhlich
Cryst. Growth Des. 2014, 14, 1018-1031. DOI:10.1021/cg4014762
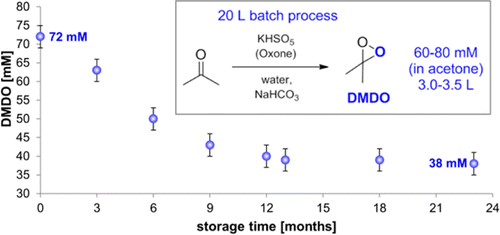
1. Practical and Efficient Large-Scale Preparation of Dimethyldioxirane [Link]
H. Mikula,* D. Svatunek, D. Lumpi, F. Glöcklhofer, C. Hametner, J. Fröhlich
Org. Process Res. Dev. 2013, 17, 313-316. DOI:10.1021/op300338q
PRESENTATIONS
Talks at conferences/symposia and universities
5th International Symposium on the Synthesis and Application of Curved Organic π-Molecules and Materials (Curo-π5), Prague, Czechia, 2023.
Talk: Concealed antiaromaticity.
11th International Conference on Materials for Advanced Technologies (ICMAT), Singapore, 2023.
Talk: Squarephaneic Tetraanhydride: A Macrocyclic Building Block for Conjugated Porous Organic Materials.
Humboldt University of Berlin, Germany, 2023, Chemisches Institutskolloquium.
Invited talk: Aromaticity and (concealed) antiaromaticity in the design of organic functional materials: Theory, synthesis, and applications in battery electrodes.
International Conference on Excited-State Aromaticity and Antiaromaticity: From Theory to Real-World Applications, Kauai, USA, 2022.
Invited talk: Aromaticity and antiaromaticity in conjugated macrocycles with locally aromatic subunits and a formal 4n π-electron system.
University of Zurich, Switzerland, 2022, Department Seminar.
Invited talk: Conjugated macrocycles as organic functional materials: From synthesis to applications.
4th International Symposium on the Synthesis and Application of Curved Organic π-Molecules and Materials (Curo-π4), Beijing, China, 2022.
Online talk: Squarephaneic Tetraanhydride: A Conjugated Macrocyclic Building Block for Redox-Active Porous Organic Materials.
Austrian Chemistry Days 2022, Vienna, Austria, 2022.
Talk: Switching between local and global aromaticity in conjugated macrocycles enables high-performance organic battery electrodes.
International Conference on the Science and Technology of Synthetic Metals 2022 (ICSM2022), Glasgow, UK, 2022.
Invited talk: Conjugated macrocycles for high-performance organic battery electrodes.
19th International Symposium on Novel Aromatic Compounds (ISNA19), Warsaw, Poland, 2022.
Talk: Aromaticity switching in conjugated macrocycles enables high-performance organic battery electrodes.
Rising Stars Conference 2022, University of Freiburg, Germany, 2022.
Talk: Conjugated macrocycles for high-performance organic battery electrodes.
Imperial College London, UK, 2021, Department Seminar.
Invited online talk: π-Conjugated macrocycles for high-performance organic battery electrodes.
Imperial College London, UK, 2021, Soft Electronic Materials Seminar of the Centre for Processable Electronics.
Invited online talk: Synthesis, properties, and applications of π-conjugated macrocycles.
Imperial College London, UK, 2020, Sustainable Energy Materials Seminar of the Centre for Processable Electronics.
Invited online talk: Exploiting aromaticity effects in conjugated macrocycles for high-performance organic battery electrodes.
15th International Conference on Organic Electronics (ICOE2019), Hasselt, Belgium, 2019.
Talk: Room-temperature post-polymerization modification turning electron-rich into electron-poor conjugated polymers.
Chalmers University of Technology, Sweden, 2019, invited by Prof. Christian Müller.
Invited talk: Synthesis and Room-Temperature Post-Polymerization Modification of Thioalkyl-Substituted Conjugated Polymers.
Synthesis of Organic Materials Symposium, Vienna, Austria, 2018.
Invited talk: Short Synthetic Routes to π-Conjugated Compounds for Organic Electronics and Beyond.
Bioelectrochemistry and more, an International Workshop, Wiener Neustadt, Austria, 2018.
Short talk: Synthesis of Dithioalkyl-substituted Poly(p-phenylene vinylene)s.
Austrian Chemistry Days, Salzburg, Austria, 2017.
Talk: Preparation of Cyanated Aromatic Compounds from Quinones.
13th International Symposium on Functional π-Electron Systems (Fπ-13), Hong Kong, China, 2017.
Talk: Rational molecular design of cyanoarenes: A new synthetic method paving the way for cyanated functional materials.
Imperial College London, UK, 2017, invited by Prof. Martin Heeney.
Invited talk: Synthesis of Cyanoarenes from Quinones: Paving the Way for Cyanated Functional Materials.
Bioelectrochemistry and more, an International Workshop, Wiener Neustadt, Austria, 2016.
Short talk: Air-Stable Multi-Cyanated Acenes - A Novel Synthesis Paving the Way for Cyanated Functional Materials.
12th International Symposium on Functional π-Electron Systems (Fπ-12), Seattle, United States, 2015.
Talk: Developing continuous junction organic electronic devices - Progress from a chemical point of view.
Bioelectrochemistry and more, an International Workshop, Wiener Neustadt, Austria, 2015.
Short talk: Inversion Of Material Properties By Post-Polymerization Modification.
International Forum/Competition of Young Researchers, Saint Petersburg, Russia, 2012.
Talk: Z-(alkylthio)alkenyl-based Compounds as Organic Nonlinear Optical Materials.